Note: Browser history (back/forward) can NOT be used here.
The BP has been providing official standards for medicines since 1864 and is the only comprehensive collection of official authoritative standards for pharmaceutical substances and medicinal products in the UK, including all European Pharmacopoeia (Ph. Eur.) monographs and texts.
The BP makes an important contribution to the Medicines and Healthcare products Regulatory Agency's (MHRA) role in protecting public health by providing quality standards for UK pharmaceutical substances and medicinal products. It is an essential reference tool for all individuals and organisations involved in the pharmaceutical sector:
 BP has a broad international reach and :
BP has a broad international reach and :
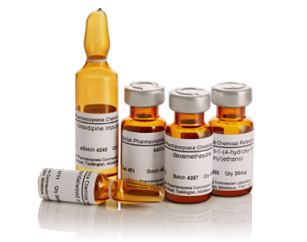
The British Pharmacopoeia Reference Chemical Substances (BPCRS) are primary standards. They are established using absolute methods and the declared contents are determined without comparison with any other substance.
BPCRS are supplied in unit packs containing sufficient material to carry out the test procedures. BPCRS are tested in our laboratory to ensure that they are suitable for use.
Download our PDF documentations